ABSTRACT
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-associated protein 9 (CRISPR-Cas9) is a promising molecular tool that has revolutionised genome editing and was recognised with the Nobel Prize in Chemistry in 2020. This study assesses the scientific productivity and knowledge structure in the scientific domain of CRISPR-Cas9 genome editing up to 2022. A total of 12,799 publications were retrieved from the Science Citation Index Expanded (SCIE) database within the Web of Science (WoS), employing related keyword searches. The records were published by authors from 107 countries in 1,731 journals. Of the total scientific publications, 499,895 total citations were found, with 39.06 average citations per publication. The United States of America dominated the research and is currently the global leader in this area with the most publications and prolific top institutions. Visualisation analysis for mapping research trends based on co-occurrences of keywords was done using VOSviewer revealing six clusters of research themes comprising; 1) conception and fundamental development; 2) gene therapy and drug delivery; 3) cancer biology; 4) plant biotechnology; 5) livestock breeding, and; 6) synthetic biology and metabolic engineering. Nanoparticle-based delivery of CRISPR-Cas9 is gaining academic attention, while CRISPR-Cas9 application in synthetic biology and metabolic engineering has progressed recently and becoming the current research interest.
INTRODUCTION
Genome editing is a technology that allows scientists to make precise, targeted changes to the DNA of an organism. The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-associated protein 9 (CRISPR-Cas9) is a specific type of genome editing technology that uses a molecule called Ribonucleic Acid (RNA), along with a nuclease protein called Cas9, to target and cut specific sequences of deoxyribonucleic acid (DNA).[1–3] The cell’s own repair mechanisms can then be utilised to add, delete, or replace specific genes or sequences of DNA.
The history of CRISPR-Cas9 in genome editing can be traced back to the discovery of the CRISPR system in bacteria. This system was initially identified as a component of the bacterial adaptive immune system and was found to function as a way for bacteria to store information about past viral infections and to use this information to quickly and effectively defend against similar future infections.[4] In 2012, Jinek et al.[1] showed that the CRISPR-Cas9 system could be used as a promising tool for genome editing at any specified DNA of interest by altering the nucleotide sequence of the guide RNA. This contribution quickly led to the development of new methods for using CRISPR-Cas9 to make precise changes to DNA and opened up new possibilities for research and various applications.[3] This discovery was so significant that it was recognised by the Nobel Prize in Chemistry in 2020, jointly awarded to Jennifer A. Doudna and Emmanuelle Charpentier.[5]
Since then, the use of CRISPR-Cas9 in genome editing has expanded rapidly, with researchers using this system to study the function of various specific genes,[3] to develop new treatments for genetic diseases,[6] and to make improvements in crops and livestock.[7,8] It has become one of the most widely used and versatile genome editing tools available and continues to drive discoveries and advances in biotechnology. Previous bibliometric studies have analysed gene editing[9] and the CRISPR system[10] in general, with no specific report on genome editing using CRISPR-Cas9. Despite the growing interest in CRISPR-Cas9 genome editing, quantitative data are non-existence in the profile of published research, while understanding the present state of the art in this knowledge domain is essential in planning future research efforts.
Analysing scientific discipline using the scientometric approach enables researchers to quantitatively examine a particular scientific field’s productivity and evolutionary nuances, simultaneously revealing the emerging areas in that field. The present paper attempts to explore the profile of global scientific publications related to CRISPR-Cas9 genome editing using scientometric analysis by summarising the scientific production and publication patterns. Secondly, this work aims to elucidate the scientific research structure and trend.
METHODOLOGY
Data source
The scientometric data source was retrieved on 20 March 2023 from the Science Citation Index Expanded (SCIE) database within Clarivate Analytics’ Web of Science (WoS) Core Collection. For inclusion criteria, the timespan was set from 1970 to 2022. The following advanced search query was used; TS=((CRISPR AND Cas9) AND (genome OR gene*) AND (edit* OR engineer* OR manipulat* OR modif*)) AND PY=(1970-2022), where TS is the topic, and PY is the publication year. The asterisk was used in the search query to include possible associate terms (i.e., genes, editing, engineering, manipulation, manipulating, modification, modifying). Document types were further restricted to the original research article (Article) and review article (Review) only. On the day of data retrieval, the WoS database was last updated on 19 March 2023.
Data analysis
The analysis was done on the same day of data retrieval to avoid bias due to periodical database updates.[11] Retrieved data was imported to a spreadsheet in Microsoft Excel 2019 to reveal research patterns regarding scientific output characteristics. Data on Total Publications (TP), Total Citations (TC), and h-index were referred directly to the database. Average citations per publication were expressed as TC to TP ratio (TC/TP). Data for co-authorship networking and thematic keyword appearance was imported as .txt format. Co-authorship network and keywords co-occurrence analyses were conducted using VOSviewer version 1.6.19[12] by setting the threshold frequency as ten (n ≥ 10).
RESULTS AND DISCUSSION
The scientific output of CRISPR-Cas9 genome editing
Figure 1 presents the number of publications of the related documents from 2012 to 2022. Based on the retrieved records, the publication on CRISPR-Cas9 genome editing was first reported in 2012 with two published pioneering studies. Therefore, documents between 2012 to 2022 were examined to assess the
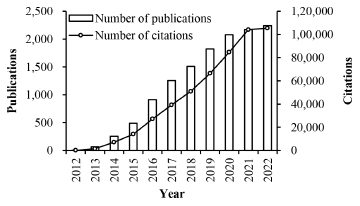
Figure 1:
The number of publications and citations per year from 2012 to 2022
relevant research output. The first paper, reported by Jinek et al. in 2012, highlighted the potential of the CRISPR system for programmable gene targeting, guided by Cas9 endonuclease derived from Streptococcus pyogenes.[1] In the same year, another work published by Gasiunas et al. demonstrated that the similarly guided endonuclease from Streptococcus thermophilus could also be programmed to target a specified DNA sequence.[13]
Overall, a total of 12,799 publication records were retrieved from the SCIE using the search query described in the methodology, in which 10,233 records were original research articles, and the remaining 2,566 were review articles. The number of publications surpassed 1,000 records since 2017 and peaked at 2,249 documents in 2022. Of the total of 12,799 documents, 499,895 total citations were recorded. From 2018 onwards, the citation counts exceeded 50,000 and recorded the highest in 2022 with 105,294 citations. All the records have an average citation of 39.06 per item and an h-index of 260. The original research article has an average citation of 41.29 per article, while review articles have an average citation of 30.12 per article. Given the steadily increasing propensity trend over the last years, the scientific output is predicted to continue to rise in the following years.
Distribution of highly productive countries and institutions
Based on the retrieved record, 107 countries contributed to the 12,799 publications in this study. Table 1 lists the top 10 countries that published the most papers indexed in the SCIE database. The United States of America (USA) dominated the list with a total of 5,056 publications, followed by China (3,583), Germany (982), Japan (961), and England (817). Publications from the USA alone constituted almost 39.5% of the total scientific output in CRISPR-Cas9 genome editing. Meanwhile, the USA also recorded the highest total citations, average citation per publication, and h-index compared to the other countries, making it the country with the highest research impact.
| Rank | Countries | TP | TC | TC/TP | h-index |
|---|---|---|---|---|---|
| 1 | USA | 5,056 | 324,233 | 64.13 | 238 |
| 2 | China | 3,603 | 116,566 | 32.35 | 137 |
| 3 | Germany | 988 | 38,831 | 39.3 | 85 |
| 4 | Japan | 970 | 31,117 | 32.08 | 81 |
| 5 | England | 821 | 29,582 | 36.03 | 82 |
| 6 | South Korea | 593 | 21,841 | 36.83 | 66 |
| 7 | France | 436 | 15,032 | 34.48 | 59 |
| 8 | Canada | 452 | 12,880 | 28.5 | 58 |
| 9 | India | 429 | 6,418 | 14.96 | 40 |
| 10 | Netherlands | 390 | 17,095 | 43.83 | 60 |
| Rank | Institutions (Country) | TP | TC | TC/TP | h-index |
|---|---|---|---|---|---|
| 1 | Chinese Academy of Sciences (China) | 718 | 32,614 | 45.42 | 91 |
| 2 | University of California System (USA) | 717 | 65,597 | 91.49 | 110 |
| 3 | Harvard University (USA) | 531 | 115,465 | 217.45 | 127 |
| 4 | University of Chinese Academy of Sciences (China) | 344 | 14,894 | 43.3 | 63 |
| 5 | Howard Hughes Medical Institute (USA) | 305 | 70,539 | 231.28 | 109 |
| 6 | Massachusetts Institute of Technology (USA) | 303 | 85,588 | 282.47 | 101 |
| 7 | Udice French Research Universities (France) | 309 | 10,968 | 35.5 | 53 |
| 8 | Chinese Academy of Agricultural Sciences (China) | 257 | 7,081 | 27.55 | 47 |
| 9 | National Institutes of Health (USA) | 243 | 14,762 | 60.75 | 50 |
| 10 | Stanford University (USA) | 238 | 15,081 | 63.37 | 62 |
In Table 2, six listed institutions were affiliated with the USA, signifying their domination in the top institutions with the most contributions in this domain. Meanwhile, the top position was led by the Chinese Academy of Sciences (718), closely followed by the University of California System (717), with only one publication difference. In terms of average citations per publication, the Massachusetts Institute of Technology received the highest average citations (282.47 citations/publication), followed by Howard Hughes Medical Institute (231.28 citations/publication) and Harvard University (217.45 citations/publication) in the top three positions. The top ten institutions have an h-index ranging from 47 to 127 in this domain.
Figure 2 depicts the global country co-authorship network mapped using VOSviewer. The size of the circle denotes the number of publications of each country. Setting the threshold publication as ten (n ≥ 10), a number of 58 out of 107 countries meet the criterion, and the USA had a high centrality (Figure 2) with the highest publication records, as reflected by the circle size, followed by China. Recent comparable bibliometric studies conducted by Wei et al.[9] and Gao et al.,[10] on gene editing and the CRISPR system, respectively, also revealed that the USA is the leading country.
Figure 2 shows that the USA closely collaborated with European countries, including Austria, Czech Republic, France, Poland, Romania, Slovenia, and Switzerland. Meanwhile, multiple lines of cooperative relationships are established between other Asian nations with China. These lines of cooperation were with South Korea, Japan, Malaysia, Philippines, Thailand, Taiwan, Singapore, Iran, Saudi Arabia, and Pakistan.
Core journals and top papers
The publication in this research domain is contributed by 1,731 publication titles in the SCIE. The top ten journals (Table 3) published about 19.34% of the total 12,799 publications. The Scientific Reports journal recorded 547 publications, making
| Rank | Journals (Publisher) | Journal indicators | TP | TC | TC/TP | h-index |
|---|---|---|---|---|---|---|
| 1 | Scientific Reports (Nature) | IF: 4.997Category: Multidisciplinary Sciences | 547 | 14,877 | 22.82 | 56 |
| 2 | International Journal of Molecular Sciences (MDPI) | IF: 6.208Category: Biochemistry and Molecular Biology; Chemistry, Multidisciplinary | 363 | 3,064 | 16.84 | 31 |
| 3 | Nature Communications (Nature) | IF: 17.694Category: Multidisciplinary Sciences | 315 | 5,164 | 32.28 | 42 |
| 4 | Frontiers in Plant Science (Frontiers) | IF: 6.627Category: Plant Sciences | 241 | 978 | 8.43 | 15 |
| 5 | PLOS One (PLOS) | IF: 3.752Category: Multidisciplinary Sciences | 240 | 2,145 | 25.54 | 27 |
| 6 | Nucleic Acids Research (Oxford University Press) | IF: 19.160Category: Biochemistry and MolecularBiology | 218 | 722 | 8.8 | 14 |
| 7 | Proceedings of the National Academy of Sciences of the United States of America (National Academy of Sciences) | IF: 12.779Category: Multidisciplinary Sciences | 166 | 3,173 | 40.16 | 31 |
| 8 | ACS Synthetic Biology (ACS) | IF: 5.249Category: Biochemical Research Methods | 138 | 4,864 | 63.17 | 40 |
| 9 | Stem Cell Research (Elsevier) | IF: 1.587Category: Cell Biology; Cell and TissueEngineering;Biotechnology and Applied Microbiology | 131 | 3,672 | 49.62 | 34 |
| 10 | Plant Biotechnology Journal (Wiley) | IF: 13.263Category: Plant Sciences; Biotechnology and Applied Microbiology | 116 | 1,327 | 21.75 | 21 |
it the most productive source title and the core journal in this area with the most documents indexed in the SCIE, followed by the International Journal of Molecular Sciences (363), Nature Communications (315), Frontiers in Plant Science (241), PLOS One (240), and Nucleic Acids Research (218). At the same time, the Scientific Reports also recorded the highest h-index of 56. Regarding average citations per publication, ACS Synthetic Biology received the highest average citations of 63.17 per article. With regard to the subject categories, major articles were published in diverse categories, which were Multidisciplinary Sciences, Biochemistry and Molecular Biology, Plant Sciences, Biochemical Research Methods, Cell Biology, Cell and Tissue Engineering, and Biotechnology and Applied Microbiology, reflecting CRISPR-Cas9 wide application in various fields of genome editing.
The most frequently cited articles are listed in Table S1. Among the ten top-cited articles, five were published in Science and three in Cell. All of the highly cited papers were published in a high-impact journal with impact factors of more than 60, except two published in Nature Protocols and BMC Plant Biology with an impact factor of 17.021 and 5.260, respectively. Nevertheless, all the journals are ranked in the first quartile within their respective categories (based on 2021 Journal Citation Reports™). Four of the top ten articles were cited over 6,000 times, with the highest being 9,423 citations. Meanwhile, nine top-cited papers were authored by correspondents affiliated with USA institutions, with Feng Zhang being the corresponding author of five articles. These highly cited papers by Feng Zhang (Table S1) include works that discuss the application of CRISPR-Cas9 in manipulating eukaryotic genomes.[14–17]
While the published works of Jennifer A. Doudna and Emmanuelle Charpentier (Table S1) demonstrated the feasibility of CRISPR-Cas9 for DNA editing,[1,18] the highly-cited publications by Feng Zhang applied this revolutionary technology for multicellular gene editing in higher eukaryotic level.[14–17] Eventually, Feng Zhang and his team at the Broad Institute of MIT and Harvard were granted the proprietorship of the CRISPR-Cas9 patent (US Patent No. 869735).[19]

Figure 2:
Global country co-authorship network in CRSPR-Cas9 genome editing with publications of more than ten (n ≥ 10) visualised by VOSviewer from 2012 to 2022. A line is established when two countries have a collaborative relationship. The thickness of each line reflects the number of co-authorships between countries
The thematic cluster of CRISPR-Cas9 genome editing and prospective path
Six main clusters of research from 2012 to 2022 were identified from a total of 1,704 keywords (with a minimum frequency of ten occurrences, n ≥ 10) and are presented in Figure 3. Overall, the keyword ‘Cas9‘ had a high centrality, and ‘CRISPR‘ had a strong correlation with ‘Cas9‘. The overall keyword networks (Figure 3 (a)) reveal six main research clusters or themes related to CRISPR-Cas9 genome editing; 1) conception and fundamental development; 2) gene therapy and drug delivery; 3) cancer biology; 4) plant biotechnology; 5) livestock breeding, and; 6) synthetic biology and metabolic engineering. These diverse thematic clusters indicate the extensive application of CRISPR-Cas9 in various fields of genome editing.
The red cluster represents the general development of CRISPR-Cas9 genome editing with the occurrence of various fundamental keywords related to its mechanistic conception, such as ‘endonuclease’, ‘bacterial immunity‘, ‘Cpf1‘, ‘DNA cleavage‘, ‘target DNA‘, ‘DNA recognition‘, ‘nickases‘, ‘RNA editing‘, ‘RNA cleavage‘, and ‘RNA-guided Cas9‘. The presence of bacterial keywords ‘Staphylococcus-aureus‘ and ‘Streptococcus-thermophillus‘ indicates the research on characterising potential endonuclease[13,20] other than Cas9 derived from Streptococcus pyogenes, initially described by Jennifer A. Doudna and Emmanuelle Charpentier.[1]
CRISPR-Cas9 technology has progressed in gene therapy and drug delivery from the blue cluster, offering the potential for targeted, precise treatments for various diseases. In gene therapy, CRISPR was used to correct genetic defects or introduce new genes into cells, potentially offering a cure for genetic disorders.[6,21] In drug delivery, CRISPR-Cas9 was used to target specific cells or tissues precisely, increasing the efficacy and reducing the side effects of drugs.[22,23] CRISPR-Cas9 can also be used to develop new drug delivery systems, such as using engineered viruses (e.g., ‘adenovirus‘, ‘adeno-associated virus‘,‘lentiviral vector‘, ‘viral vectors‘) to deliver therapeutic genes to cells.[24]
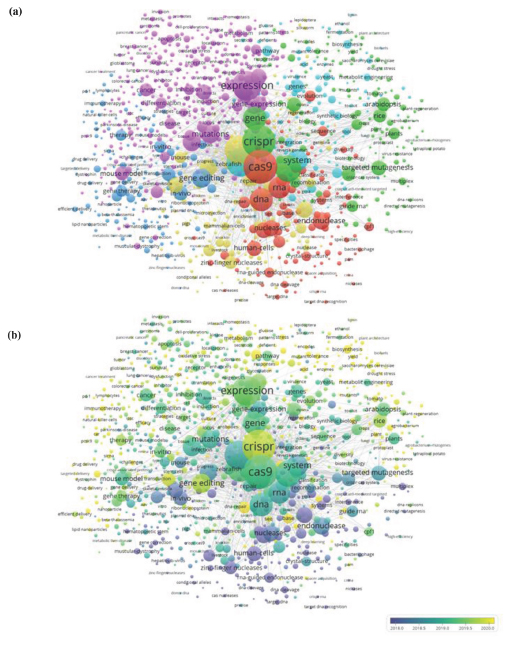
Figure 3:
Keywords co-occurrence (n ≥ 10) clusters of CRISPR-Cas9 genome editing (a) and its temporal evolution (b) visualised by VOSviewer from 2012 to 2022. The circle size indicates the frequency of occurrence of each keyword. Distance between circles denotes the degree of relatedness of each keyword. Different colours represent different thematic clusters
Next, cancer biology has also emerged as an essential discipline within CRISPR-Cas9 genome editing, shown in the purple cluster, which is also closely allied with the blue cluster. In this cluster, CRISPR-Cas9 was used to study the genes and pathways involved in cancer development and progression, potentially identifying new targets for cancer therapies.[25] Some of the keywords in the group related to the type of cancer being studied are ‘breast cancer‘, ‘cell lung cancer‘, ‘colorectal cancer‘, ‘pancreatic cancer‘, and ‘prostate cancer’. One of the significant studies in this cluster involves the precision engineering of chimeric antigen receptors-T cell (CAR-T) by CRISPR-Cas9, with the occurrence of the term ‘CAR-T‘ and ‘T cell receptors‘, for immunotherapy of oncogenic mutation.[26]
The green and yellow clusters are research themes that represent CRISPR-Cas9 applications in the field of agriculture. The green cluster is characterised as a plant biotechnology cluster that deals with manipulating plant genomes to introduce desirable traits, such as disease resistance, increased yield, and improved nutritional content.[7,27] Examples of crop-related keywords in this cluster that are being experimented with include ‘barley‘, ‘brassica‘, ‘cotton’, ‘maize‘, ‘rice‘, ‘soybean’, ‘tomato‘, and ‘wheat‘. CRISPR-Cas9 was reported to be used to modify the expression of genes involved in plant growth and development, enabling the creation of traits that are better adapted to specific environmental conditions (e.g., ‘drought stress‘, ‘salt tolerance‘, ‘climate change‘), and resistant to pathogens (e.g., ‘geminiviruses’, ‘virus resistance‘) and diseases (e.g., ‘bacterial-blight‘), hence may reduce the need for harmful pesticides and herbicides.
The yellow cluster represents the research on livestock breeding. This cluster covers gene modifications of various livestock such as ‘cattle’, ‘chicken’, ‘fish’, ‘pig’, ‘sheep‘, and ‘swine’. Similar to the green cluster where CRISPR-Cas9 has been used to introduce crop trait enhancement, it was used to develop gene drives that can spread beneficial traits throughout livestock animals, i.e., improve their health, productivity, and even ability to resist diseases.[8] On the other hand, another critical study in this cluster comprises research that knocked out genes with CRISPR-Cas9 in a one-step procedure involving microinjection at the zygotic level[28] done in variously reported mammals (i.e., ‘knockout mice‘, ‘knockout pigs‘), where the keyword ‘microinjection‘ displayed strong associations with ‘one-step generation‘ and ‘knock-out‘.
Lastly, the cyan cluster is connected to CRISPR-Cas9 application in synthetic biology and metabolic engineering, allowing researchers to precisely modify genetic material and engineer cells to perform specific functions in these two synergetic fields. [29,30] The most valuable application of CRISPR-Cas9 in this cluster is for modifying the metabolic pathways of microbial cells, enabling researchers to produce beneficial compounds. Manipulation of the genes involved in metabolic pathways precisely allows researchers to optimise the production of these compounds.[30] For instance, CRISPR-Cas9 was used to alter microbial genomes of eukaryotes (e.g., ‘Saccharomyces cerevisiae‘, ‘Pichia pastoris‘) and prokaryotes (e.g., ‘Bacillus subtilis‘, ‘Pseudomonas putida‘) for the bioconversion of microbial metabolites (e.g., ‘ethanol‘, ‘riboflavin‘).
The overlay visualisation of temporal keyword evolution is shown in Figure 3 (b), where the lightening of colour chroma signifies emerging keywords while the darker ones occur earlier. The red cluster appeared darkest compared to others, where most keywords in this group existed before 2018. Hence, this infers that the development of this technology can be regarded as fully grown or developed, and presently, the research trend is transitioning into the application stage. Analysis of the keywords implies the application of CRISPR-Cas9 in synthetic biology and metabolic engineering (cyan cluster) has progressed significantly since 2020 and becoming a recent research interest, shown by the lighter colour of the majority of keywords in this group. Meanwhile, in the blue and purple clusters, keywords related to ‘nanoparticles‘, ‘gold nanoparticles‘, ‘lipid nanoparticles‘, ‘drug delivery‘, and ‘immunotherapy‘ are also lighter in colour, which has become visible since 2020. Therefore, the emergence of these keywords indicates that nanoparticle-based in vivo delivery of CRISPR-Cas9 genome editing for drug delivery and cancer therapy is currently gaining academic attention.[22]
Overall, remarkable advancement has been made in this knowledge domain. However, the use of CRISPR also raises concerns around safety, regulation, and ethical concerns, highlighting the need for careful consideration of the potential risks of the technology. As with any new technology, it is essential to carefully consider the potential risks and benefits before using it in any applied field. Although there is no specified thematic cluster related to safety or ethics from the keywords retrieved from the SCIE database in Figure 3, a bibliometric assessment reported by Asquer and Krachkovskaya[31] based on the Social Science Citation Index (SSCI) and Arts and Humanities Citation Index (A&HI) within the WoS Core Collection, revealed that societal and humanities issues related to ethical concern, public acceptance and perception, and, technology regulation and governance were also gained much attention in CRISPR genome editing.
On the other hand, several novel genome editing based on CRISPR-associated systems with a distinctive promising feature is being explored using nucleases other than Cas9, such as Cas12 (formerly known as Cpf1) and Cas13 (formerly known as C2c2) nucleases.[32] Therefore, future work should explore the scientific productivity of CRISPR genome editing research that employs these nucleases. In the meantime, it is important to note that the data presented in this study is limited to the SCIE database only. Future research should also consider evaluating the scientific production and research trends of this area concurrent with scientometric data sources retrieved from the Scopus database, which covers a greater volume of publications.[33] A simultaneous complimentary evaluation of both databases may give a more comprehensive overview of the past and current development of CRISPR-Cas9 genome editing.
CONCLUSION
This work provides a brief overview of the status of pertinent research linked to CRISPR-Cas9 genome editing. A total of 12,799 documents were retrieved from the SCIE, recorded from 107 countries in 1,731 journals. Given the growing tendency over the study period, it is envisaged that related scientific production will continue to expand. The current work briefly explored the status of CRISPR-Cas9 genome editing research and provided a valuable outline of the global state of this domain. Six main clusters of research themes were identified, where CRISPR-Cas9 application in synthetic biology and metabolic engineering has progressed recently. Nanoparticle-based delivery of CRISPR-Cas9 in drug delivery and cancer therapy is becoming the current research interest. Complimentary assessment with the Scopus database should be conducted in future studies for a comprehensive framework of this scientific domain.
Cite this article
Halmi MFA, Zulkifli MAF, Zaman KHK. CRISPR-Cas9 Genome Editing: A Brief Scientometric Insight on Scientific Production and Knowledge Structure. J Scientometric Res. 2023;12(2):395-402.
References
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, et al. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-21. [Google Scholar]
- Ma Y, Zhang L, Huang X. Genome modification by CRISPR/Cas9. The FEBS Journal. 2014;281(23):5186-93. [Google Scholar]
- Janik E, Niemcewicz M, Ceremuga M, Krzowski L, Saluk-Bijak J, Bijak M, et al. Various aspects of a gene editing system—CRISPR–Cas9. International Journal of Molecular Sciences. 2020;21(24):9604 [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709-12. [Google Scholar]
- Uyhazi KE, Bennett J. A CRISPR view of the 2020 Nobel Prize in Chemistry. The Journal of Clinical Investigation. 2021;131(1) [Google Scholar]
- Hussain W, Mahmood T, Hussain J, Ali N, Shah T, Qayyum S, et al. CRISPR/Cas system:a game changing genome editing technology, to treat human genetic diseases. Gene. 2019;685:70-5. [Google Scholar]
- Hamdan MF, Karlson CKS, Teoh EY, Lau SE, Tan BC. Genome editing for sustainable crop improvement and mitigation of biotic and abiotic stresses. Plants. 2022;11(19):2625 [Google Scholar]
- Jabbar A, Zulfiqar F, Mahnoor M, Mushtaq N, Zaman MH, Din AS, et al. Advances and Perspectives in the Application of CRISPR-Cas9 in Livestock. Molecular Biotechnology. 2021;63(9):757-67. [Google Scholar]
- Wei X, Pu A, Liu Q, Hou Q, Zhang Y, An X, et al. The bibliometric landscape of gene editing innovation and regulation in the worldwide. Cells. 2022;11(17):2682 [Google Scholar]
- Gao C, Wang R, Zhang L, Yue C. Visualisation analysis of CRISPR gene-editing knowledge map based on CiteSpace. Biology Bulletin. 2021;48:705-20. [Google Scholar]
- Sun J, Yuan BZ. Bibliometric mapping of top papers in Library and Information Science based on the Essential Science Indicators Database. Malaysian Journal of Library and Information Science. 2020;25(2):61-76. [Google Scholar]
- Van Eck NJ, Waltman L. Software survey:VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523-38. [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proceedings of the National Academy of Sciences. 2012;109(39) [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819-23. [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 2013;8(11):2281-308. [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84-7. [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology. 2013;31(9):827-32. [Google Scholar]
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213) [Google Scholar]
- Hom L. Patent pools for CRISPR technology. Science. 2017;355:274 [Google Scholar]
- Chen W, Zhang Y, Yeo WS, Bae T, Ji Q. Rapid and efficient genome editing in Staphylococcus aureus by using an engineered CRISPR/Cas9 system. Journal of the American Chemical Society. 2017;139(10):3790-5. [Google Scholar]
- Zhang B. CRISPR/Cas gene therapy. Journal of Cellular Physiology. 2021;236(4):2459-81. [Google Scholar]
- Duan L, Ouyang K, Xu X, Xu L, Wen C, Zhou X, et al. Nanoparticle delivery of CRISPR/Cas9 for genome editing. Frontiers in Genetics. 2021;12:673286 [Google Scholar]
- Hasanzadeh A, Noori H, Jahandideh A, Moghaddam HN, Mousavi KSM, Nourizadeh H, et al. Smart strategies for precise delivery of CRISPR/Cas9 in genome editing. ACS Applied Bio Materials. 2022;5(2):413-37. [Google Scholar]
- Xu CL, Ruan MZ, Mahajan VB, Tsang SH. Viral delivery systems for CRISPR. Viruses. 2019;11(1):28 [Google Scholar]
- Zhang H, Qin C, An C, Zheng X, Wen S, Chen W, et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Molecular Cancer. 2021;20:1-22. [Google Scholar]
- Razeghian E, Nasution MK, Rahman HS, Gardanova ZR, Abdelbasset WK, Aravindhan S, et al. A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Research and Therapy. 2021;12(1):1-17. [Google Scholar]
- Gan WC, Ling AP. CRISPR/Cas9 in plant biotechnology:applications and challenges. BioTechnologia. 2022;103(1):81-93. [Google Scholar]
- Navarro-Serna S, Vilarino M, Park I, Gadea J, Ross PJ. Livestock gene editing by one-step embryo manipulation. Journal of Equine Veterinary Science. 2020;89:103025 [Google Scholar]
- García-Granados R, Lerma-Escalera JA, Morones-Ramírez JR. Metabolic engineering and synthetic biology:synergies, future, and challenges. Frontiers in Bioengineering and Biotechnology. 2019;7:36 [Google Scholar]
- Singh R, Chandel S, Ghosh A, Dey D, Chakravarti R, Roy S, et al. Application of CRISPR/Cas system in the metabolic engineering of small molecules. Molecular Biotechnology. 2021;63:459-76. [Google Scholar]
- Asquer A, Krachkovskaya I. A bibliometric analysis of research on CRISPR in social sciences and humanities. Re:GEN Open. 2021;1(1):14-23. [Google Scholar]
- Yan F, Wang W, Zhang J. CRISPR-Cas12 and Cas13:the lesser known siblings of CRISPR-Cas9. Cell Biology and Toxicology. 2019;35:489-92. [Google Scholar]
- AlRyalat SS, Malkawi LW, Momani SM. Comparing bibliometric analysis using PubMed, Scopus, and Web of Science databases. Journal of Visualized Experiments. 2019;152 [Google Scholar]

